Pharmaceutical Computer Validation Introduction Manual And Cd Gmp Good Manufacturing Practices Training Introduction To Meet Fda Regulations In The On Computer System Validation And Part 11

Analytical Instrument Qualification And System Validation Pharmaceutical Computer Validation Introduction Manual And Cd Gmp Good Manufacturing Practices Training Introduction To Meet Fda Regulations In The On Computer System Validation And Part 11
pdfslide.net

Who Guideline Validation Computerized Systems Appendix5 Qas16 Pharmaceutical Computer Validation Introduction Manual And Cd Gmp Good Manufacturing Practices Training Introduction To Meet Fda Regulations In The On Computer System Validation And Part 11
www.scribd.com

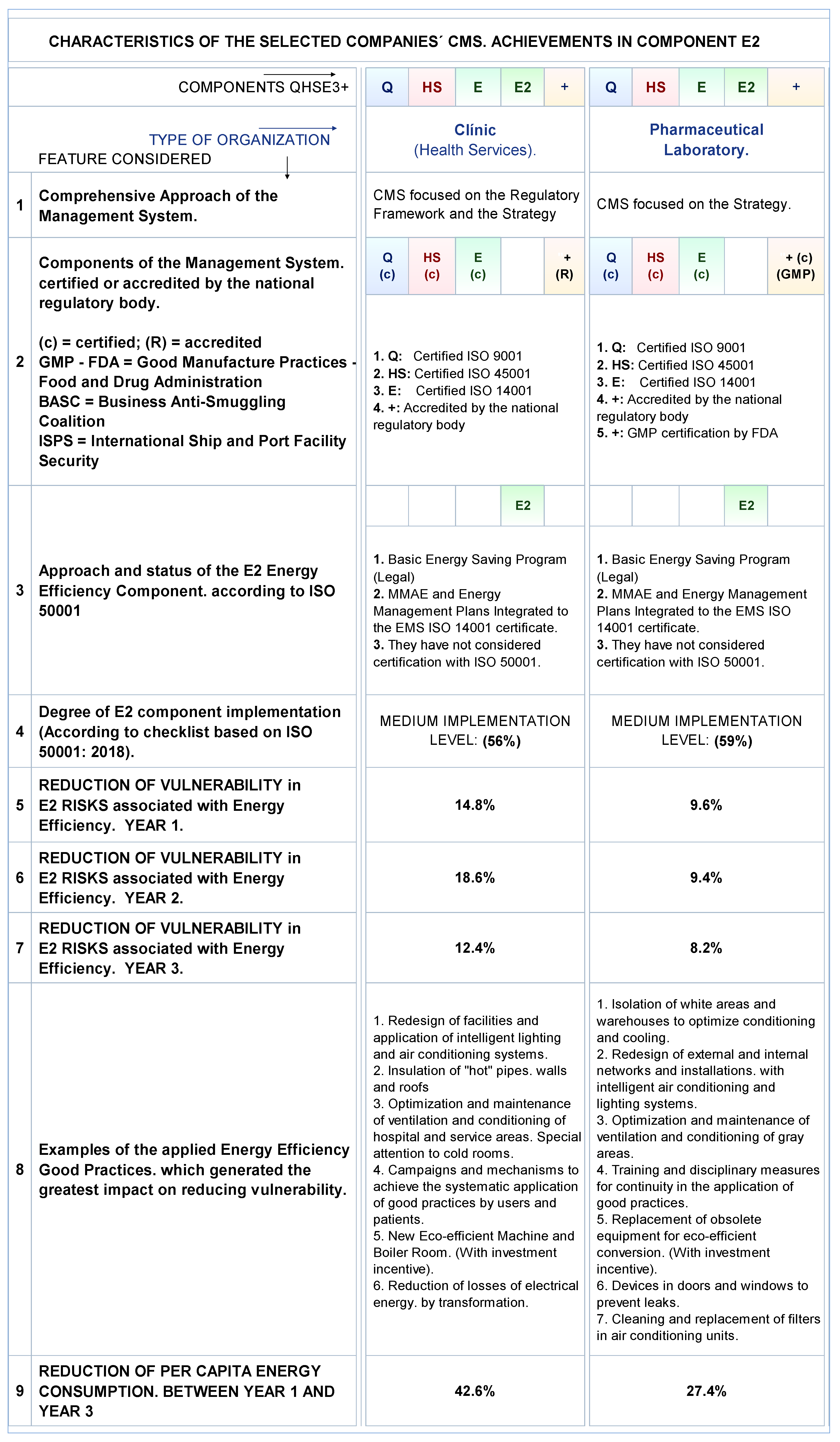
Energies Free Full Text Iso 50001 2018 And Its Application In Pharmaceutical Computer Validation Introduction Manual And Cd Gmp Good Manufacturing Practices Training Introduction To Meet Fda Regulations In The On Computer System Validation And Part 11
www.mdpi.com
More from Pharmaceutical Computer Validation Introduction Manual And Cd Gmp Good Manufacturing Practices Training Introduction To Meet Fda Regulations In The On Computer System Validation And Part 11
- Dna Deamination And The Immune System Aid In Health And Disease Molecular Medicine And Medicinal Chemistry
- Badai Siklon Tropis Yang Terjadi Di Australia Disebut Badai
- Das Andere Babybuch
- Chaste And Chastised These Crimes Against Chastity Wont Go Unpunished
- Moby Dick Gli Adelphi
- Congratulations On Your College Graduation Images
- Badak Putih Terakhir
- Hidden Object I Spy Games For Kids
- Millennium Development Goals And Community Initiatives In The Asia Pacific By Amita Singh Eduardo T Gonzalez Stanley Bruce Thomson
- Fortnite Season Chapter 2 Season 3 Victory Umbrella
- Uñas Rojas Decoradas 2020 De Moda
- Tokyo Esp 07
- Collection 1 Of Grand Mother Story Books
- Suku Osing Banyuwangi Santet
- Battle Royale Fortnite Chapter 2 Season 3 Skins Battle Pass










